At the Interface Between Man and Machine
A Sneak Peak of Bespoke Bodies: The Design & Craft of Prosthetics
Bespoke Bodies: The Design and Craft of Prosthetics is our latest book being published here at CoDesign, which is now available for pre-order. Included in this issue of Design Museum Magazine is an excerpt from the book, which highlights the incredible surgical innovations of Dr. Shriya Srinivasan and her team, who have developed an unprecedented technique for amputating limbs.

Photo by John Soares
By Dr. Shriya Srinivasan, PhD, Co-written by Sabaat Kareem
About Dr. Shriya Srinivasan Dr. Shriya Srinivasan is a Schmidt Science Fellow and Junior Fellow at the Harvard Society of Fellows. She graduated from Case Western Reserve University with a BS in biomedical engineering, with a concentration in biomaterials. Shriya received her doctoral degree in medical engineering and medical physics through the Harvard-MIT Health Sciences and Technology program in January 2020. Her doctoral research focused on the development of novel neural interfaces utilizing tissue engineering to better interface human limbs with prostheses, in the context of amputation and paralysis. She developed the Regenerative Agonist-antagonist Myoneural Interface (AMI) that will ultimately enable patients to control their prosthesis with native neural signals. She also explored optogenetic techniques to create novel strategies to accelerate and improve neural control. Shriya has been awarded the Delsys Prize and the Lemelson-MIT Student Prize for her innovative work.
Shriya was a former director of MIT Hacking Medicine and works passionately on global health projects. Shriya is currently designing devices for gastrointestinal neuromodulation in the MIT Langer Lab in collaboration with Dr. Giovanni Traverso. In her spare time, she professionally dances Bharatanatyam, a south Indian classical dance form, and co-directs Anubhava Dance Company.
Surgical Amputation’s Slow Evolution
Life-saving amputations are accompanied by a vast set of emotional and physical consequences. Prostheses and rehabilitative therapies have evolved greatly over the last few decades to help amputees readjust. Despite the immense technological and scientific advancements achieved in our time, the actual technique for surgical amputation has remained largely static.In fact, the procedure has hardly changed over the last 200 years.
A team of researchers at the MIT Media Lab and Brigham and Women’s hospital, including Drs. Hugh Herr, Matthew Carty, Tyler Clites and I, used interdisciplinary thinking to reimagine the amputation process, test it on the benchtop, validate the working mechanisms, and implement it in human beings. This amputation methodology specifically focuses on restoring the communication pathways between the brain, the peripheral limbs, and prostheses.
The Need for Sensory Feedback
We know that sensory feedback from our skin and joints plays a central role in our development, communication, motor control, and psychosocial well-being. The body’s innate ability to sense the position, speed, and forces of our limbs enables balance, fine motor control, and trajectory planning. As a professional dancer, this sensation, called proprioception, is something I’m critically reliant upon; knowing where my hands and feet are, how quickly they are moving and the forces acting upon them helps my body automatically execute jumps and turns at the right time, with the right power. It allows my dancing to be fluid, timed with the music, and free from my visual attention.
Proprioception is orchestrated by specialized receptors in muscle pairs, called agonist-antagonist muscle pairs, which work together to signal the brain. With current amputation techniques, these pairings are severed, leaving the patient with little proprioception after amputation. This makes it difficult to operate even the smartest prostheses and heavily limits mobility, decreasing the daily quality of life for persons with limb loss.
Over the past four years, my doctoral work utilized the lens of engineering to redesign amputation techniques to restore the sense of feeling and enable enhanced communication with prostheses. This was targeted not only at patients who were undergoing amputations for the first time, but also for patients who had already undergone amputations, giving them a chance to “upgrade” their residual limb’s functionality.
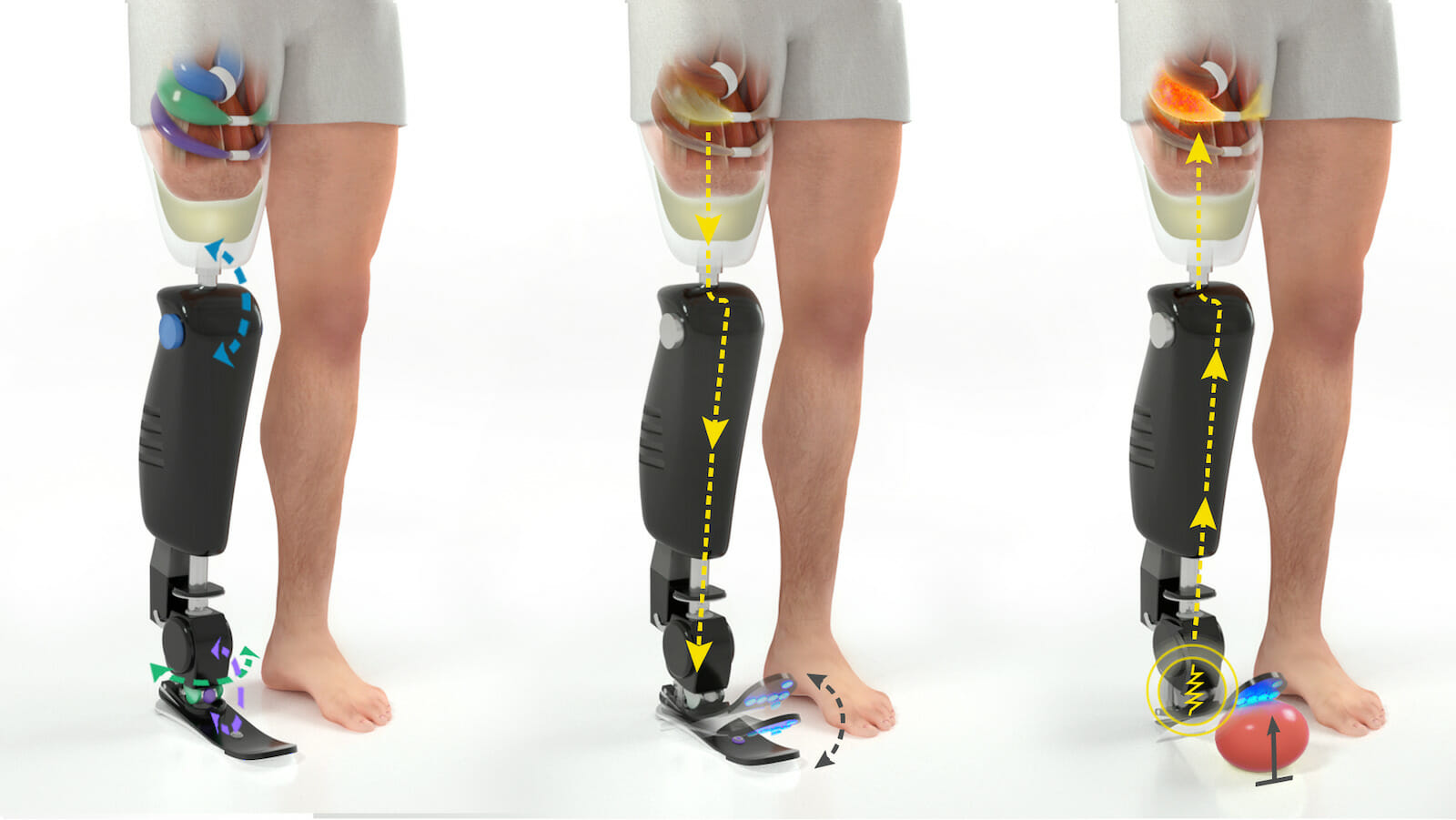
Preserving joint motion and sensation: The Agonist-Antagonist Myoneural Interface (AMI) is comprised of an agonist and antagonist muscle pair connected in such a way that contraction of the agonist muscle induces extension of the antagonist. For an above-knee amputation, 3 AMIs are constructed for the knee (blue), subtalar (purple), and ankle (green) joints and positioned in the thigh.
A Design Framework
The design of new architectures for sensory feedback, through grafting, regenerative, and “spare-parts” surgical techniques, represents a novel approach to bridge the communication between the human body and synthetic devices. Utilizing biological tissues as the “hardware,” in terms of sensors and actuators offers valuable advantages. Tissues possess high energy density, autonomous repair and regeneration, biocompatibility, mechanical robustness, and the incorporation of highly-evolved mechanotransducers. Their sensing and signaling infrastructure are resilient to surgical manipulations. By leveraging mechanotransduction, these organs circumvent the need to synthetically encode complex sensory data and transmit it with specificity into thousands of nerve channels. As a biomedical engineer, entering the realm of surgical innovation, I was excited to apply these principles to address the challenges faced by individuals with amputation.
Proprioception is the body’s natural way of relaying information about one’s position in space to the nervous system. During an amputation procedure, the agonist (contracting) and antagonist (relaxing) muscle pairs, responsible for proprioception, are decoupled, making it difficult for the person to sense their phantom limb’s or a prosthesis’ position. When we move, the agonist and antagonist muscles stretch and contract, sending electrical signals to our nervous system and allowing us to sense our bodies in space. The AMI procedure reconnects those muscles during amputation in order to save proprioception. With an AMI, when an individual moves his/her leg, their AMI muscles contract and stretch just like the muscles originally would have. The AMI muscles send the natural electrical signal to the nervous system and inform the body of the leg’s position.
What is the Problem?
Since the late 1800s, the surgical procedure for amputation has largely remained unchanged. The surgery entails making a circular cut around the entirety of the limb and severing the muscles, nerves, and bone at the amputation site. The residual muscles are then wrapped distally to create ample soft tissue padding for comfortable prosthetic socket use. Since the muscles are buried differently for each patient and sometimes deep within the residual limb, the ability to send EMG (electromyographic) signals to command a prosthesis are limited. Often, muscles will contract together or not contract at all, making it difficult to decode a patient’s intention. Thus, even with the most sophisticated prostheses, patients struggle to command them. Patients also don’t receive any sensory feedback, which limits mobility.
What’s the Solution?
We designed the agonist-antagonist myoneural interface (AMI), a system which reinstates agonist-antagonist muscle pairs, to enable proprioceptive feedback. During a primary elective amputation, native agonist-antagonist pair muscles are surgically connected by the tendons and channeled through a tunnel created on the bone, functioning like a pulley. Contraction of the agonist stretches the antagonist and gives rise to the sensory feedback signals.
These muscles have the capacity to move individually and signal to a bionic prosthesis more clearly the way in which they’d like the prosthesis to move. In addition, stimulation from the prosthesis to the muscles can help inform the individual where the prosthesis is in space and how it’s moving. Throughout all of these processes, the agonist-antagonist muscle pairing works to send natural signals back to the brain regarding the phantom or prosthetic limb.
The AMI muscles mimic the function of the agonist/ antagonist muscles. When an amputee moves their leg, the stretching and contracting of the AMI muscles send electrical signals to the brain and to the prosthesis. The brain can then calculate how much force should be applied to the movement as well as the location of the prosthesis.
From the Benchtop to the Bedside
Translating our method to the clinic, over 25 patients to date have received AMIs during amputation through a clinical trial at the Brigham and Women’s Hospital in Boston, MA. Functional testing demonstrates that these patients have significantly greater sensation of their phantom limbs and much greater phantom mobility. When paired with bionic prostheses, they are also able to control them more intuitively, with greater resolution, and receive feedback about the prostheses’ movements.
While these functional results were promising, I was still very curious as to how the brain received and integrated the signals of proprioception from the limbs. It’s well-known, and easy to imagine, that losing a limb causes significant changes in the brain, some of which are thought to cause phantom pain. As such, I wanted to know whether preserving limb physiology could also help preserve the brain’s structure and functioning. Thus, we embarked on a journey that involved imaging the brains of patients with the AMI and comparing them to patients with traditional amputation and no amputation. This was a journey into unchartered waters for many reasons: our lab had never done such imaging studies, very few labs had imaged the brains of patients with amputation, and very little is known about proprioception in the brain.
Comparing the brain activity in regions associated with proprioception, we resolved to determine how exactly the AMI worked and if we could preserve more brain anatomy and function with the new amputation method. Following hundreds of hours of functional magnetic resonance imaging (fMRI) and data analyses, we were amazed by the results. In the area of the brain responsible for sensory feedback, patients with AMI amputation had no difference from patients without amputation, while patients with traditional amputation had a significant decrease. Moreover, we saw that the brains of AMI subjects were relatively unchanged to the normal brain!
The journey to this point was a colossal collaborative effort. It couldn’t have been possible without the futuristic thinking of Hugh, the technical prowess of Matt, and the tireless and creative work of Tyler, other graduate students, and me. Seeing the improvement in patients’ lives, their transformation from a life of limited mobility and chronic pain to one of much greater mobility and psychological freedom was incredibly rewarding. Using a design framework that holistically captured the continuum between the person and the machine revealed not only scientific advances and technological evolution, but also benefits for patients’ daily experiences and quality of life. Thinking about the valuable effect of reengineering hundreds of years of clinical habit motivates me to make translational research a responsibility for the biomedical engineer; to question the status quo and to urge the community to make clinical care parallel and incorporate the technological advances we have.

When Engineering Meets Surgery: Shriya with collaborators Dr. Matthew Carty (pictured in the first image) and Hugh Herr (pictured here).
Harnessing Regeneration
Unlike most machines, the human body has the capacity to regenerate function as cells die or lose functionality. With the right cues and structure, even nerves can be nurtured to regrow into the desired targets to enable signaling and neural communication. Putting a spare-parts methodology to work again, I designed the regenerative AMI–a surgical process in which we take nerves that are normally orphaned during amputation and have them grow into small muscle grafts. These grafts take on the function of the nerve’s original target. By connecting these grafts with their agonist-antagonist partners, we can recreate original signaling pathways to restore sensory feedback and communication with prostheses. Working with small animal models and cadavers, we designed and tested this system. What is impressive about the technique is that a person with an above-knee amputation can still maintain control of their knee, subtalar, and ankle joints and receive sensory feedback from all three! It’s also a rather simple surgical process that can be done not only in well-resourced hospitals, but also in battlefields and low-resource settings. Through a collaborative effort, we recently implemented this procedure in the first human subject and have a larger clinical trial underway.
Next Steps
Based on what we are learning, we are continuing to refine our methods, gather more data, and disseminate this information to the broader biomedical and surgical communities for widespread implementation. The success of the AMI stemmed largely from transdisciplinary collaboration. Historically, there has been a disconnect between the fields of medical engineering and clinical medicine. Our team was able to adopt a holistic design process, harness diverse expertise, and learn from each other. In doing so, we have come farther than I would have ever expected. Notwithstanding, there’s a long road ahead and we are eager to continue the journey.
Following my graduation, I’ve started a postdoctoral fellowship at the MIT Langer Lab, where I’ll be acquiring expertise in medical device design and tissue engineering. Ultimately, I hope to merge these skills with my passion for re-engineering physiology to restore functionality in cases of disease and dysfunction, to seamlessly blend the powers of biology and technology, and to make the line between man and machine ultimately invisible.

A world of difference: Shriya works with Madison, who received an above-knee amputation incorporating AMIs, to characterize her limb’s functionality and signaling capabilities as well as her brain’s perception.
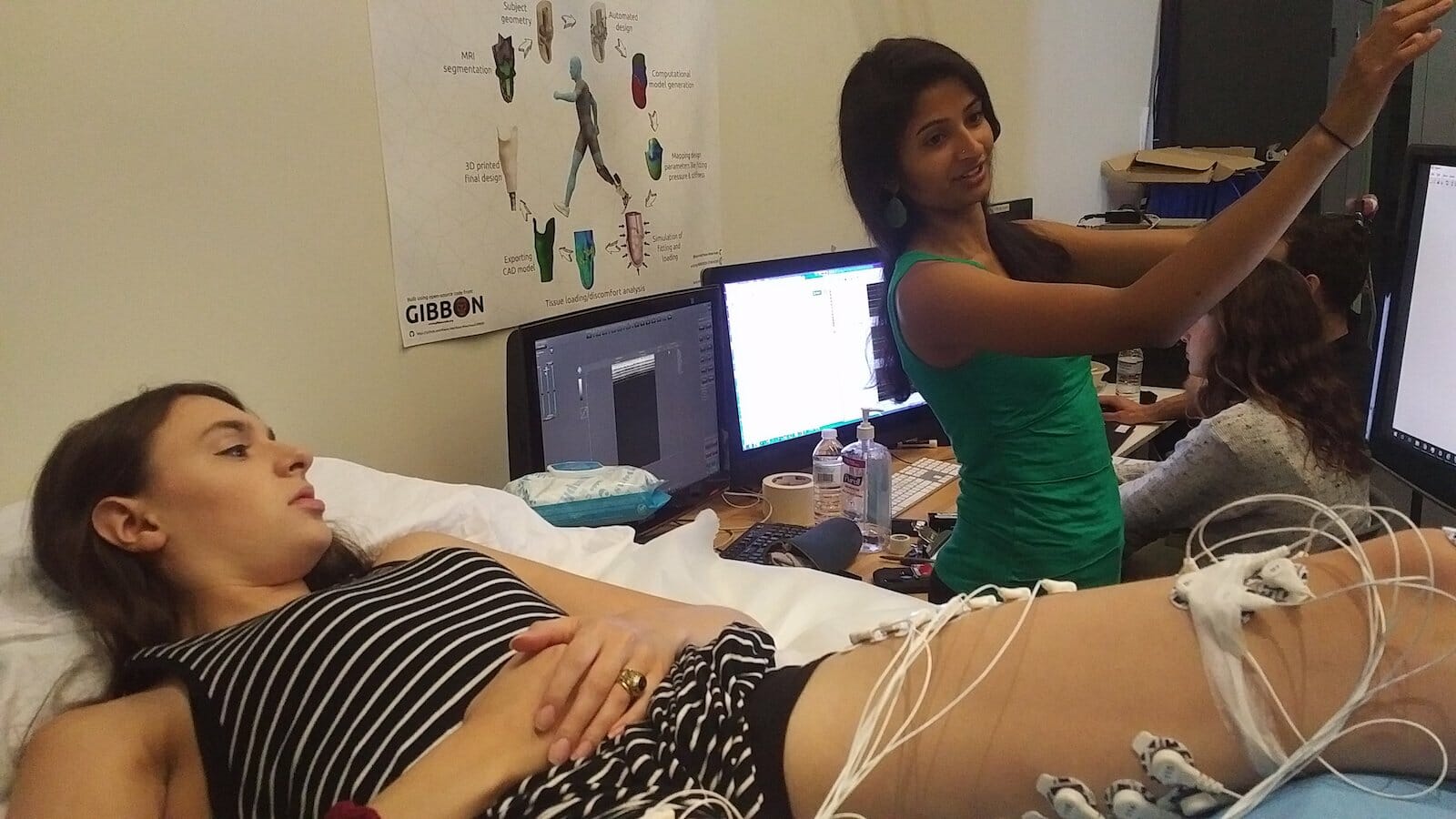
Dr. Srinivasan works with her patients on functional testing in a lab setting.
From Design Museum Magazine Issue 017
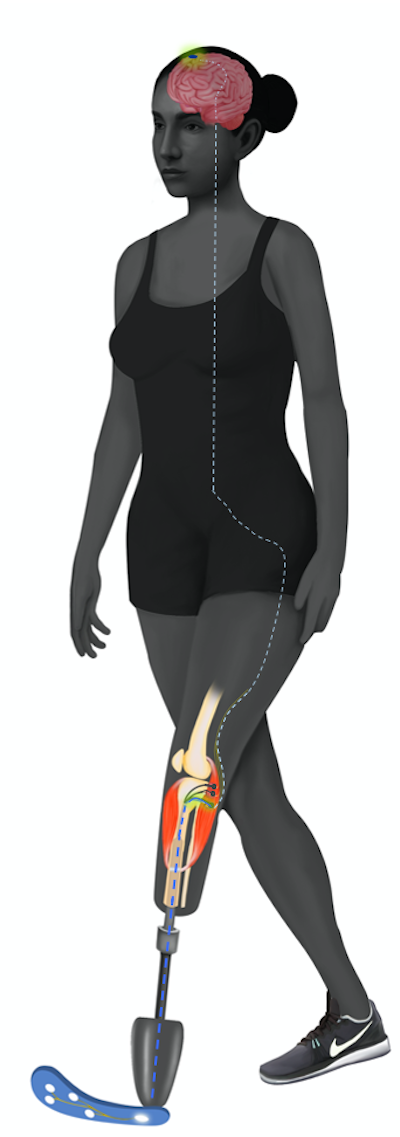
What does the brain perceive? Amputation normally leads to reorganization and sometimes the loss of brain structures responsible for movement and sensation of the given limb. A study of the brain’s motor and sensory circuitry carried out by Dr. Srinivasan and the team reveals that the AMI amputation paradigm can preserve sensory feedback signals in the brain and restore more normal sensorimotor connections.
